 1
1
2,2,6,6-Tetramethylpiperidine is a hindered amine light stabilizer that is low in toxicity and highly efficient. It contains reactive groups that can be bonded to polymer chains, resulting in improved performance. It does not easily volatilize during processing and does not affect the original color of the material. It is compatible with most industrial solvents and significantly enhances the light and oxidative aging resistance of polyethylene plastics, fiber products, and polypropylene materials, surpassing conventional UV absorbers and quenchers [1].
![Figure 1: Synthesis route of 2,2,6,6-tetramethylpiperidine [2]. Figure 1: Synthesis route of 2,2,6,6-tetramethylpiperidine [2].](https://imgen3.guidechem.com/img/answer/2023/9/4/1693817973234273.jpg)
Figure 1: Synthesis route of 2,2,6,6-tetramethylpiperidine [2].
The HPLC pump and receiver with a cold trap are connected to a microreactor. The reactor is heated in a nitrogen flow (50 ml/min) in an oven to 250°C. Once this temperature is reached, an incremental amount of hydrogen is mixed into the N2 flow until the proportion of hydrogen is 100%. The temperature is then raised for 30 minutes to 350°C and subsequently lowered to 100°C. At this temperature, THTMP of Example A1 is added at a rate of 4.2 ml/h using the pump. Hydrogen is simultaneously added at a rate of 50 ml/min. The reaction is quantitatively completed at 110-130°C. The total yield of 2,2,6,6-tetramethylpiperidine obtained in the form of a colorless liquid is actually 100%. 2,2,6,6-Tetramethylpiperidine, yield 100%. The synthesis route is shown in Figure 1.
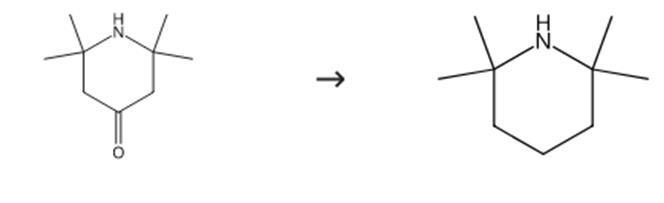
Figure 2: Synthesis route of 2,2,6,6-tetramethylpiperidine.
Hydrazine hydrate (1.0 L, 1.0 kg, 21 mol) is added to liquid triacetone amine (mp 34-38°C; 2.0 kg, 13 mol) within 30 minutes. Due to moderate exothermic reaction, the temperature temporarily rises to 90°C. The viscous but uniform brown mixture is kept at 60°C in a bath and potassium hydroxide (48 g, 0.85 mol) in a solution of diglycol (0.25 L) and paraffin oil (60 mL) is carefully dripped into it at a rate of 2.5 g/min using a pump (model ProMinent Electronic A2001). The solution is maintained at 210°C (±10°C) in a polyethylene glycol bath (see Figure 1). The formed 2,2,6,6-tetramethylpiperidine and released H2O are continuously removed through a 50 cm Vigreux column by distillation and collected in a separation funnel until the reaction is terminated. The organic layer obtained by distillation yields 2,2,6,6-tetramethylpiperidine. It is a colorless liquid with a yield of 1.51 kg (83%). bp 155-157°C; nD20 1.4454 [raised to nD20 1.4555]; d420 is 0.837. The synthesis route is shown in Figure 2.
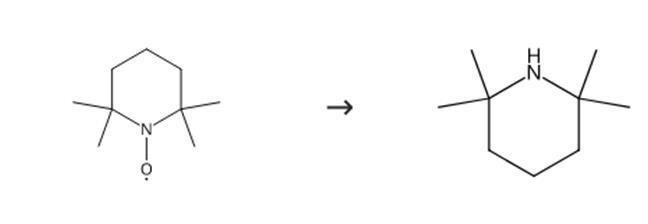
Figure 3: Synthesis route of 2,2,6,6-tetramethylpiperidine.
2,2,6,6-Tetramethyl-4-oxapiperidine-1-oxyl (10 mmol), excess ethanethiol (approximately 5 ml/1 g nitroxide), and benzene (approximately 2.5 ml/1 ml ethanethiol) are mixed. After completion of the reaction (solution turns colorless), the mixture is extracted with 1 M hydrochloric acid. The acidic layer is carefully alkalized with potassium carbonate. The acidic layer is extracted with dichloromethane. The organic layer is dried with magnesium sulfate. The organic layer is filtered. The organic layer is evaporated. The purified product (distillation, crystallization, sublimation, or chromatography) yields the titled compound 2,2,6,6-tetramethylpiperidine.
[1] Cai, Z., Ding, L. (2021). Synthesis method of 2,2,6,6-tetramethylpiperidine. Plastic Additives, (02), 36-37+58.
[2] Zakrzewski, J. (1990). A reaction of nitroxides with ethyl mercaptan. A mild method for the conversion of nitroxides into their corresponding amines. Monatshefte fuer Chemie, 121(10), 803-8.

